From simulating protocol variations for trial design to predicting relapse rates in patients, we take a look at how AI-powered simulations are improving clinical trial efficiency and success.
AI-powered simulations are changing the way the pharmaceutical industry develops and tests new drugs.
Tech providers are racing to develop simulations — which can replicate real-world scenarios in clinical trials — that can predict drug interactions, patient responses, and potential side effects.
Below, we’ll highlight a few areas where AI-powered simulations are being used to enhance clinical trials and research across:
- Patient outcomes in randomized controlled trials
- Cancer cell behavior for drug design
- Predicting relapse rates in patients
- Protocol variations for trial design
Simulating patient outcomes in randomized controlled trials
AI simulations can transform randomized controlled trials (RCTs) by creating “digital twins” (virtual replicas) of patients. By simulating control groups, patient digital twins enable researchers to predict with accuracy how patients might respond to treatment without exposing individuals to potential side effects.
This can also streamline the trial process by reducing the need for large numbers of human participants and shortening the time it takes to conduct traditional RCTs. AI-powered digital twins also allow for rapid, virtual trial iterations and early detection of adverse effects, potentially bringing new treatments to market more quickly.
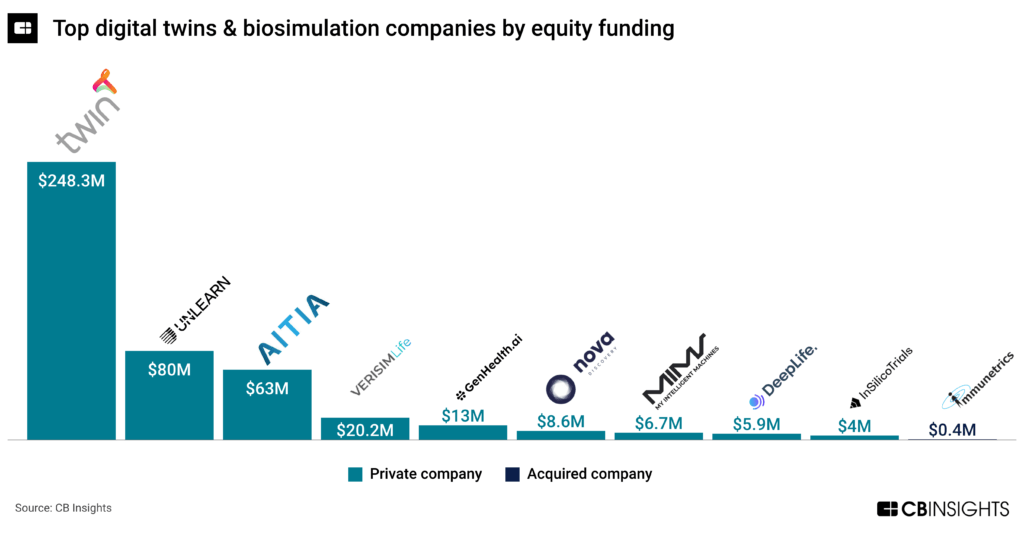
The most well-funded company in the digital twins and biosimulation market is Twin Health, with nearly $250M in equity funding, followed by Unlearn at $80M. Twin Health secured another $50M in December 2023 as it zeroes in on chronic metabolic diseases.
Want to see more research? Join a demo of the CB Insights platform.
If you’re already a customer, log in here.