Caris Life Sciences
Founded Year
2008Stage
Loan | AliveTotal Raised
$1.714BValuation
$0000Last Raised
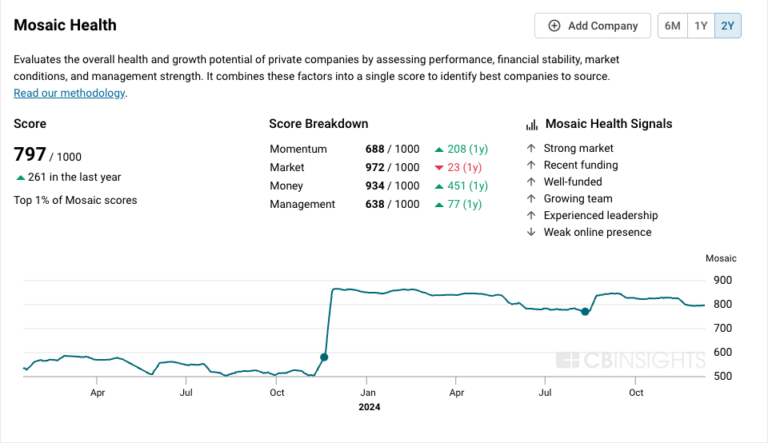
$400M | 2 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+1 points in the past 30 days
About Caris Life Sciences
Caris Life Sciences provides molecular profiling services, including DNA and RNA sequencing, to supply oncologists with information about cancer at the molecular level, facilitating personalized treatment plans. The company also offers biopharmaceutical solutions, assisting in the discovery, clinical development, and commercialization of new cancer therapies. It was founded in 2008 and is based in Irving, Texas.
Loading...
ESPs containing Caris Life Sciences
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The liquid biopsy cancer screening market offers a non-invasive alternative to traditional tissue biopsies for detecting cancer. This market includes various solutions such as circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) tests, which can detect cancer at an early stage and monitor treatment effectiveness. The liquid biopsy method is less invasive, faster, and more cost-effectiv…
Caris Life Sciences named as Outperformer among 15 other companies, including Exact Sciences, QIAGEN, and Veracyte.
Loading...
Research containing Caris Life Sciences
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Caris Life Sciences in 4 CB Insights research briefs, most recently on Dec 11, 2024.
Aug 22, 2022 report
State of Biopharma Tech Q2’22 Report
May 26, 2022
Where are the next US tech hubs?Expert Collections containing Caris Life Sciences
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Caris Life Sciences is included in 3 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,270 items
Precision Medicine Tech Market Map
160 items
This CB Insights Tech Market Map highlights 160 precision medicine companies that are addressing 9 distinct technology priorities that pharmaceutical companies and healthcare providers face.
Oncology Tech
463 items
This collection includes companies applying technology to cancer care, diagnosis, and treatment. Examples include vendors offering cancer detection and diagnosis, oncology clinical decision support, real-world data, and AI oncology drug discovery.
Caris Life Sciences Patents
Caris Life Sciences has filed 1 patent.
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
3/6/2018 | 3/9/2021 | Monoclonal antibodies, Molecular biology, Clusters of differentiation, Immunology, Biotechnology | Grant |
Application Date | 3/6/2018 |
|---|---|
Grant Date | 3/9/2021 |
Title | |
Related Topics | Monoclonal antibodies, Molecular biology, Clusters of differentiation, Immunology, Biotechnology |
Status | Grant |
Latest Caris Life Sciences News
Mar 20, 2025
News provided by Share this article Share toX A 295,000+ patient study revealed variable efficacy among cancers for patients treated with tissue-agnostic drugs and uncovered the potential for expanding approvals to other drugs of the same class IRVING, Texas, March 20, 2025 /PRNewswire/ -- Caris Life Sciences ® (Caris), a leading next-generation AI TechBio company and precision medicine pioneer, today announced the publication of " Real-world evidence provides clinical insights into tissue-agnostic therapeutic approvals " in Nature Communications. Utilizing the largest real-world clinical and genomic dataset of tissue-agnostic indications reported to date across more than 295,000 patients, the study provides a comprehensive evaluation of real-world outcomes for patients eligible for these powerful "pan-cancer" therapies, such as pembrolizumab and larotrectinib. These drugs are categorized as tumor-agnostic therapies, targeting specific genetic or molecular features of tumors rather than their anatomical location or histology. The study focused on the use and clinical benefits of tissue-agnostic therapies, which target specific molecular biomarkers across numerous cancer types. Key findings include: More than 20 percent of patients are candidates for a tissue-agnostic drug. Approximately five percent of patients who lacked any tumor-specific indication were found to carry a tissue-agnostic indication and became eligible for tissue-agnostic drug treatment. Tissue-agnostic therapy uptake was poor for rare indications, illustrating the need for enhanced clinical education. Current tissue-agnostic indications could potentially be expanded, as evidenced by clinical benefits in tumor types and drugs of the same class that were not investigated in the original clinical trials. "By harnessing the power of Caris' large clinico-genomic dataset, we have shown that tissue-agnostic drugs produce different outcomes in different tissues," said George W. Sledge, Jr., MD , EVP and Chief Medical Officer of Caris and study author. "We additionally found evidence of clinical benefit beyond approved indications, illustrating the enormous potential of real-world data to inform the clinical use of tissue-agnostic drug approvals, potentially improving patient outcomes." "Caris has generated one of the most extensive clinico-genomic datasets in oncology through years of comprehensive molecular profiling," said Caris President and study author David Spetzler, MS, PhD, MBA . "Our advanced analyses of real-world data now enable us to uncover findings of clinical value for large patient populations. One in five patients in our study were eligible for a tissue-agnostic drug, which demonstrates the widespread impact of our findings." Detailed Scientific Information This study used a real-world database of 295,316 molecularly profiled tumor samples with associated clinical outcomes data across 57 tumor types to investigate the utility and uptake of six tissue-agnostic therapies (pembrolizumab, larotrectinib, entrectinib, dostarlimab, dabrafenib and selpercatinib). At least one current FDA-approved tissue-agnostic indication (TMB-High, MSI-High/dMMR, BRAFV600E mutations, and NTRK and RET fusions) was detected in 21.5% of patients, including 5.4% lacking any tumor-specific indication. In patients with rare NTRK fusions, there was poor clinical uptake of the clinically successful targeted therapies larotrectinib and entrectinib, underlining the need for oncologist education on rare indications. Strikingly, significant differences in pembrolizumab-associated outcomes were observed across tumor types for the most common indications (TMB-High and MSI-High/dMMR), demonstrating that the effects of these therapies are not tissue-agnostic. Clinical benefits were also observed in tumor types and drugs of the same class (such as nivolumab) that were not investigated in pivotal clinical trials. This suggests the possible expansion of therapeutic avenues for a given tissue-agnostic indication. These findings demonstrate the power of Caris' extensive real-world dataset for generating valuable clinical insights to inform the clinical implementations of tissue-agnostic drug approvals and improve outcomes for patients with cancer. Designed to capture and analyze molecular information from tissue and blood in a comprehensive manner, the proprietary Caris Platform is a powerful set of precision medicine solutions, providing Whole Exome Sequencing (WES) and Whole Transcriptome Sequencing (WTS) as standard practice. Caris sequences at sector-leading depth of coverage, which directly correlates to increased accuracy and detection of low-frequency biomarkers of relevance. Caris also evaluates protein biomarkers through an extensive menu of immunohistochemical (IHC) tests analyzed in a tumor-type specific manner, which in combination with WES and WTS, provides a comprehensive view of a patient's disease across DNA, RNA and proteins. The Caris approach reveals an individualized molecular blueprint of a patient's disease, providing actionable, personalized treatment pathways and driving superior clinical outcomes for patients. About Caris Life Sciences Caris Life Sciences® (Caris) is a leading next-generation AI TechBio company and precision medicine pioneer that is actively developing and delivering innovative solutions to revolutionize healthcare and improve the human condition. Through comprehensive molecular profiling (Whole Exome and Whole Transcriptome Sequencing) and the application of advanced AI and machine learning algorithms, Caris has created the large-scale, multimodal database and computing capability needed to analyze and further unravel the molecular complexity of disease. This convergence of sequencing power, big data and AI technologies provides a differentiated platform to deliver the next generation of precision medicine tools for early detection, diagnosis, monitoring, therapy selection and drug development. Caris was founded with a vision to realize the potential of precision medicine in order to improve the human condition. Headquartered in Irving, Texas, Caris has offices in Phoenix, New York, Cambridge (MA), Tokyo, Japan and Basel, Switzerland. Caris or its distributor partners provide services in the U.S. and other international markets. To learn more, please visit CarisLifeSciences.com . Caris Life Sciences Media:
Caris Life Sciences Frequently Asked Questions (FAQ)
When was Caris Life Sciences founded?
Caris Life Sciences was founded in 2008.
Where is Caris Life Sciences's headquarters?
Caris Life Sciences's headquarters is located at 750 West John Carpenter Fwy, Irving.
What is Caris Life Sciences's latest funding round?
Caris Life Sciences's latest funding round is Loan.
How much did Caris Life Sciences raise?
Caris Life Sciences raised a total of $1.714B.
Who are the investors of Caris Life Sciences?
Investors of Caris Life Sciences include OrbiMed Advisors, Braidwell, Sixth Street Partners, T. Rowe Price, Neuberger Berman and 15 more.
Who are Caris Life Sciences's competitors?
Competitors of Caris Life Sciences include Syapse and 6 more.
Loading...
Compare Caris Life Sciences to Competitors

Genialis is an RNA biomarker company that focuses on advancing translational research and cancer drug development within the healthcare sector. The company specializes in discovering RNA biomarkers using machine learning, which aids in positioning oncology drugs, stratifying clinical trials, and developing diagnostic tests to predict patient responses and guide treatment decisions. Genialis collaborates with pharmaceutical and diagnostics companies to deliver precision medicine solutions that aim to improve clinical outcomes. It was founded in 2015 and is based in Boston, Massachusetts.

GenomOncology is a software company that focuses on cancer care through data analysis. The company provides software solutions that process healthcare data into treatment options and insights, aimed at supporting oncology workflows. GenomOncology serves healthcare institutions and molecular labs, offering tools for data enablement, pathology, decision support, and analytics. It was founded in 2012 and is based in Cleveland, Ohio.

OncoHealth is a digital health company that provides a platform for oncology value management and virtual cancer care. The platform includes nursing, mental health, nutrition, and resource navigation services for patients and healthcare providers. OncoHealth serves health plans, employers, providers, and patients in the cancer care ecosystem. It was founded in 2009 and is based in Atlanta, Georgia.

CureMatch is a healthcare technology company that specializes in precision oncology and digital decision support solutions for cancer treatment. The company offers services that analyze genomic biomarkers to match patients with personalized cancer treatments, utilizing AI algorithms to rank combination therapy options. CureMatch primarily serves oncologists and cancer treatment centers seeking to implement precision medicine strategies. It was founded in 2015 and is based in San Diego, California.

CancerIQ is a platform that focuses on cancer prevention and early detection within the healthcare sector. The company provides software and services that assist in risk assessment, patient navigation, care plan management, and analytics for healthcare providers. CancerIQ's solutions serve oncology and genetics, breast centers, and preventive care sectors, allowing for cancer risk programs and integration with clinical workflows. It was founded in 2013 and is based in Chicago, Illinois.

Medidata specializes in providing a unified platform for clinical research within the life sciences sector. The company offers a range of products and solutions designed to streamline clinical trials, including data management, clinical operations, and patient engagement technologies. Medidata's platform serves various sectors, including biopharma, medical device companies, and academic research organizations. It was founded in 1999 and is based in New York, New York.
Loading...